When Depression Doesn’t Improve With Medication
Depression is a serious medical condition—not something a person can simply overcome through willpower alone. It can disrupt everyday life, affecting energy levels, concentration, sleep, relationships, and overall wellbeing.
While antidepressant medications can be beneficial for some, others may find them ineffective, difficult to tolerate due to side effects, or inconsistent with their personal treatment preferences.
For individuals facing these challenges, noninvasive, medication-free therapies such as repetitive Transcranial Magnetic Stimulation (rTMS) may be worth considering. rTMS works by delivering repeated magnetic pulses to targeted areas of the brain that are known to play a role in mood regulation, without circulating drugs throughout the body.
Major Depressive Disorder affects millions of individuals each year, and leading medical institutions such as the Mayo Clinic note that depression is linked to measurable changes in how the brain functions.
Research indicates that certain brain networks involved in mood, motivation, and emotional regulation may operate differently in people experiencing depression.
Depression is common in both active-duty service members, veterans, and their families.
If you feel stuck, you are not alone. And there are additional treatment options available.
Transcranial Magnetic Stimulation (TMS) is designed to influence these specific brain pathways using targeted magnetic pulses delivered from outside the body. Because it does not require surgery or systemic medication, TMS may be an appealing option for individuals seeking alternatives to traditional pharmacological treatment.
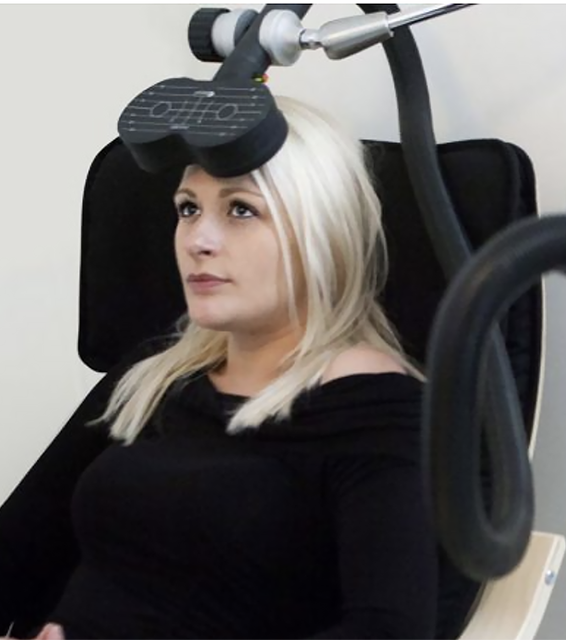
What is rTMS?
rTMS (Repetitive Transcranial Magnetic Stimulation) uses gentle magnetic pulses to stimulate areas of the brain associated with mood regulation.
The treatment is:
- Non-invasive
- Performed while you are awake
- Completed in 20–40 minute sessions
- No recovery time required
Most patients return to normal activities immediately after each session.
Independent Research Studies on the effectiveness of TMS for Depression
TMS, including its repetitive form (rTMS), has received FDA clearance for the treatment of Major Depressive Disorder. An expanding body of peer-reviewed research suggests that rTMS can significantly reduce depressive symptoms, particularly in individuals who have not achieved adequate results with medication alone.
Ongoing clinical studies continue to investigate how different stimulation techniques, treatment schedules, and individualized protocols may further improve outcomes and expand its clinical applications.
The following section highlights selected meta-analyses, systematic reviews, and clinical trials that examine the use of rTMS in the treatment of depression.
TMS Should Be Considered as First-Line Treatment For Moderate to Severe Major Depressive Disorder, in Psychiatric News, October 2022.
The article’s author, Richard A. Bermudes, reviewed a decade’s worth of studies on the effectiveness of TMS, and wrote this:
“As I read the [APA] guidelines recently and considered the number of new outcome studies conducted with TMS, I believe TMS should be considered, in addition to pharmacotherapy and psychotherapy, as a first-line treatment for patients with moderate to severe major depressive disorder.”
A retrospective chart review to assess the impact of alpha- guided transcranial magnetic stimulation on symptoms of PTSD and depression in active-duty special operations service members, 21 June 2024.
“This data provides a demonstration of significant reduction in PTSD and depression symptoms and safety with the application of a-rTMS in active-duty special operations military personnel. Expansion of targeted neuromodulation programs could be impactful for military and civilian populations.”
Efficacy of repetitive transcranial magnetic stimulation (rTMS) adjunctive therapy for major depressive disorder (MDD) after two antidepressant treatment failures July 2023.
“Several meta-analyses proved the efficacy of rTMS treatment in MDD, which is comparable to pharmacotherapy, and may have even better tolerability. Our results strengthened that rTMS is associated with clinically relevant antidepressant effect in TRD as well, and may be a beneficial tool in the add-on treatment of patients with TRD. Furthermore, rTMS adjunctive treatment to antidepressants was found to be specifically effective in achieving full remission.”
Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of quality of life outcome measures in clinical practice, July 2013.
This study found: “Conclusion: These data confirm that TMS is effective in the acute treatment of MDD in routine clinical practice settings. This symptom benefit is accompanied by statistically and clinically meaningful improvements in patient-reported QOL [quality of life] and functional status outcomes.”
Repetitive Transcranial Magnetic Stimulation for the Treatment of Resistant Depression: A Scoping Review. June 2022.
“Overall, 16 out of the 17 studies suggested that rTMS treatment was effective, safe and tolerated in TRD. For patients with TRD, rTMS appears to provide significant benefits through the reduction of depressive symptoms…”
The association between sample and treatment characteristics and the efficacy of repetitive transcranial magnetic stimulation in depression. Oct 2022.
“Data from nearly 3000 patients from 65 randomized controlled trials were included in this meta-analysis. rTMS was confirmed as efficacious in treating depression when looking at symptom reduction, response and remission.”
Efficacy of repetitive transcranial magnetic stimulation in treatment-resistant depression: the evidence thus far, August 2019.
“rTMS is rapidly gaining popularity as a treatment modality for depression. There is growing evidence to support its use in patients with depression as a monotherapy or as adjunct with pharmacotherapy. Additionally, rTMS has been found to be safe and effective in pregnant patients and elderly patients…”
Use of Transcranial Magnetic Stimulation for Depression, May 2019.
“The clinical efficacy of TMS as an antidepressant has been well established. TMS is an innovative and promising treatment modality for patients with TRD [treatment-resistent depression].”
Experimental depression treatment is nearly 80% effective in controlled study, October 28, 2021.
“In a double-blind controlled study, high doses of magnetic brain stimulation, given on an accelerated timeline and individually targeted, caused remission in 79% of trial participants with severe depression.
“A new type of magnetic brain stimulation brought rapid remission to almost 80% of participants with severe depression in a study conducted at the Stanford University School of Medicine.
Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice, Carpenter et al, 2012.
This study found as its conclusion: “These data indicate that TMS is an effective treatment for those unable to benefit from initial antidepressant medication.”

Does TRICARE Cover rTMS?
Yes. TRICARE covers FDA-approved rTMS for Major Depressive Disorder when a patient meets medical necessity criteria.
You may qualify if:
- You have been diagnosed with depression
- You have tried antidepressant medications without adequate relief
- A physician determines that rTMS is appropriate
Our team will verify your benefits and guide you through the approval process.
You do not have to navigate it alone.
What Happens Next?
- Submit the short form below
- We verify your TRICARE benefits
- Schedule your consultation
- Begin treatment if approved
Clear. Direct. No pressure.

Check Your TRICARE Eligibility
Complete this form, and our team will contact you to review your coverage and next steps. There is no obligation, and your data is secure.
Frequently Asked
Does TRICARE really cover rTMS?
Yes, for Major Depressive Disorder when eligibility criteria are met.
Are there side effects?
rTMS is generally well tolerated, with mild scalp discomfort or headache that dissipates being the most common side effects.
Is rTMS safe?
Yes. rTMS is FDA-approved as a treatment for depression and has been safely used for over a decade.
Does it hurt?
It is painless. Most patients report a light tapping sensation on the scalp.

Physician-Led Depression Care in Glenview
Your evaluation and treatment are overseen by a licensed physician experienced in neuromodulation and depression care. Every patient receives a comprehensive medical assessment to determine whether TRICARE-covered rTMS is appropriate before treatment begins.
“After a month of treatment, I was grateful to be depression-free and medication-free for the first time in a very long time.”
—Veteran after rTMS treatment
FDA-approved | TRICARE Accepted | Non-Invasive
You’ve Served. Now Let’s Focus on Your Healing.
If depression hasn’t improved with medication, TRICARE-covered rTMS may be your next step.